Educational content on VJHemOnc is intended for healthcare professionals only. By visiting this website and accessing this information you confirm that you are a healthcare professional.
In this case study on follicular lymphoma, learn more about:
✓ Approaching diagnosis and disease classification
✓ The contemporary approach to the treatment of FL
✓ The relevance of mutation testing for patients with FL in the era of personalized therapy
Case presentation: A 62-year-old man presents with multicompartmental lymphadenopathy above and below the diaphragm. A core needle biopsy of a representative left cervical lymph node is performed.
- Histologic sections of the lymph node biopsy showed partial architectural effacement by lymphoma. The neoplasm had an entirely follicular pattern; the follicles lacked polarization and well-formed mantle zones. (image not shown)
- The cytologic composition of the follicles is variable, comprised of a mixture of centrocytes and centroblasts, the majority in the range of what would be considered grade 3A by WHO R4th edition (Fig 1A). Mitotic figures are readily identifiable in the grade 3A follicles.
- Immunohistochemical studies performed on the lymph node biopsy show the neoplastic cells are positive for CD10 (Fig 1B), CD20, BCL-6, and BCL2 (Fig 1C) and are negative for cyclin D1. The Ki-67 proliferation rate is ~50% (Fig 1D). (Histologic images courtesy of Dr. L. Jeffrey Medeiros, MD Anderson Cancer Center).
- The most recent iteration of the WHO classification (WHO 5th, PMID 35732829) has eliminated the requirement for follicular lymphoma grading. It has modified the classification and terminology used for follicular lymphoma (Fig 2-3).
Figure 1
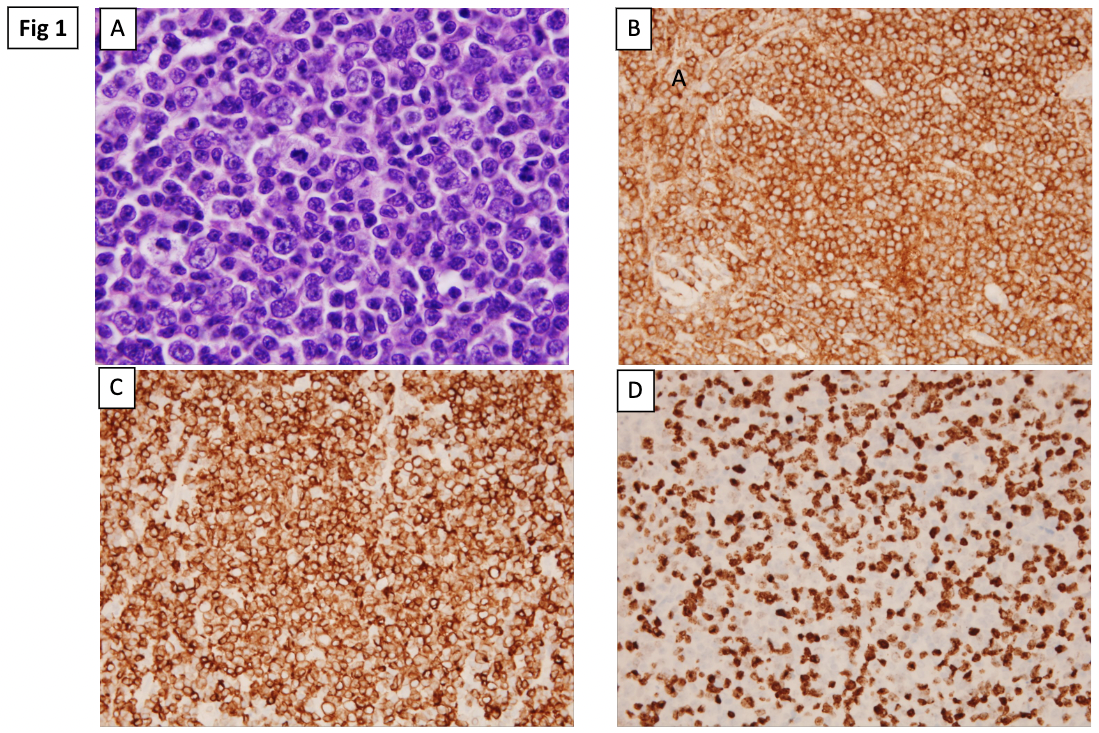
Figure 2: Follicular lymphoma subclassification, WHO 5th ed

Figure 3: Follicular lymphoma subclassification, WHO 5th ed cont.

Dr. Sanam Loghavi:
Hello, my name is Sanam Loghavi. I’m a hematopathologist and molecular pathologist, at MD Anderson Cancer Center. And I’m here with my colleague, Dr. Loretta Nastoupil, who is a hemoncologist focusing on lymphoma, also at MD Anderson Cancer Center. Great to be here with you.
Dr. Loretta Nastoupil:
Pleasure is mine.
Dr. Sanam Loghavi:
Thank you. So we are here today to I guess talk about updates in follicular lymphoma, mostly as they pertain to pathology, because I’m a pathologist. So as you know very well, and our audience I’m sure is aware, follicular lymphoma grading has been changed in the new version of the WHO, but it remains the same in the ICC, which is the International Consensus Classification system. So with elimination of grading, which just to give a little bit of a history or context to the audience. Before, when we made a diagnosis of follicular lymphoma, we were also providing a grade to the treating physician, that was really based on the number of centroblasts and centricites that we saw microscopically in the biopsy that was provided to us. So now, that has been eliminated for a couple of reasons in the WHO. One was that reproducibility was not great among pathologists, so two pathologists could look at the same biopsy and come up with a different grade.
And then the other was that between maybe grade two and grade grade three A, there wasn’t much of a management change based on the grade that was provided. So in the new version of the WHO, it was thought that maybe it’s just better to eliminate grading and go with other criteria, with the exception of grade three B, which is considered more akin or similar to a diffuse large B-cell lymphoma, because you just have sheets of centroblasts. The other grades have really been eliminated. So how does that impact your practice, if at all? Tell me, what do you think about grading?
Dr. Loretta Nastoupil:
I think you have to recognize I have the pleasure of working with exceptional hematopathologists that can make that fine distinction. If you think about oncology in community practice where a lot of patients with follicular lymphoma are actually going to be seen and treated, I think the nuance of distinguishing between the grade probably is harder, or less impactful than it is in terms of deciding on a treatment option. We’ve never really known if grade should determine treatment, and partly we’ve not had studies well done that were really defined based off of grade. Some of the trials that used a non-anthrocycline-based treatment just excluded grade three.
Some of the retrospective data suggested that grade three may be better served with an anthrocycline because of that fine line of distinguishing large cell lymphoma from follicular. But I think in the modern era where we are even questioning, do you need chemotherapy at all in follicular lymphoma? I think that’s where grading probably is not as critical to discern. But I think you make a really good point. We probably should still be clearly separating out those with three B follicular lymphoma, because we probably would approach them differently.
Dr. Sanam Loghavi:
Of course, that’s very helpful for us to know is what you care about. And so when you tell me that at least a subset of patients with follicular lymphoma are now not getting chemotherapy at all, how are you treating them?
Dr. Loretta Nastoupil:
I think there’s still great debate on this point, but we conducted the relevant study which looked at lenalidomide in combination with rituximab, which was a non-chemo option. We included eligibility for grade one, two, or three A. Which again, was a little bit intriguing, because you have the experimental arm that doesn’t contain chemotherapy. The other arm did allow for three different chemotherapy backbones, CVP, bendamustine, and CHOP. So even though it’s 2023, we’re still debating what is the preferred chemo backbone, and there are a lot of nuances, but we’ve never shown that one single treatment’s going to really have a major impact on overall survival. So that then opens the door, do you need chemotherapy at all? And so I think now as we have things like lenalidomide, rituximab, some of the bispecifics moving into frontline therapy, I think that really does then open the door for alternatives that are not chemotherapy-based.
Dr. Sanam Loghavi:
Yeah, I mean this is fantastic, right? Because if you think about follicular lymphoma, it’s such an indolent disease with a long course, that these patients, if you give them chemotherapy, they get exposed to a lot of chemotherapy over time. So it’s really fabulous that you’re saving these patients from chemotherapy.
So I have one other question for you, in the era of modern medicine and molecular pathology, we’re obviously sequencing a lot of our tumors, especially in the heme malignancy field, but I think the vast majority of laboratories probably don’t have the bandwidth to sequence every biopsy that they get. So if you wanted to prioritize sequencing for patients with follicular lymphoma, who are the patients that benefit the most at this time, given the treatment options that you have, from sequencing and from knowing their DNA and mutation profile? And then what are the mutations that you care about and why? What are the treatment implications?
Dr. Loretta Nastoupil:
Really important questions, and I think the reason why we’ve lagged a little bit behind in terms of the molecular profiling and that having an impact on treatment, is because most of our patients do well. So we don’t have that unmet need of identifying that subgroup that’s going to do very poorly with the available treatment, so then we could either design studies or more rationally target those subgroups. And so we have the luxury of riches. And so I also think if we’re in a center where you have limited resources, and you’re trying to decide how you’re going to use them with sequencing a follicular lymphoma case, who’s likely going to respond to just about anything you throw at them, it’s probably not a valuable use of your resources.
But then the flip side of that coin is, we’re never going to move the field forward if we don’t interrogate tissues and understand more about the underlying biology, and really those cases where you have really disparate outcomes. Because you’ll have some patients that you could observe for 10 to 14 years and they never progress, versus others that get intensive chemoimmunotherapy and are progressing quickly through multiple lines of therapy.
My brain tells me those are two different cases, even though we may label them as the same follicular lymphoma. So we probably need to do a better job at centers like ours, really sequencing those poor-risk cases, or those poor outcomes, so that we can understand those, and then contrast those to those really good outcomes where patients don’t even need any treatment, and still have a normal life expectancy. So that we can then inform the general clinician when and who to sequence.
I think actionable right now, we know we have an EZH2 inhibitor in the form of tazemetostat, where your response rate’s going to be double if you have an EZH2 mutation, but it doesn’t suggest that you can’t use it in a patient with wild-type, because they still get a meaningful PFS.
Dr. Sanam Loghavi:
Okay.
Dr. Loretta Nastoupil:
So as of today, I think if you’re thinking about tazemetostat and response matters to you, it might be very helpful to sequence the tumor. If you have a patient where the side effect profile is just much more attractive and you don’t have good alternatives, you could even use tazemetostat in the absence of knowing the EZH2 mutation status. So that makes it really challenging.
Dr. Sanam Loghavi:
And is that paid by insurance, for wild-type status as well? That’s fantastic. All right, that’s very good. Any other recommendations for practicing pathologists? Anything that you think will help improve patient care for patients with follicular lymphoma, from where you stand?
Dr. Loretta Nastoupil:
I think we all need to work together better as a community, and do a better job of biopsying patients when they relapse. I think that’s one area. And again, it’s a discussion with patients-
Dr. Sanam Loghavi:
Of course.
Dr. Loretta Nastoupil:
And if you’ve had someone who’s been biopsied multiple times in the last few months, do we really need to subject them to another invasive procedure? But on the flip side of the coin, if you have a patient where it’s not behaving like follicular lymphoma, we need to biopsy those cases, so we know that it’s still follicular and not another alternative diagnosis or transformation, because our treatment’s going to vary quite dramatically.
Dr. Sanam Loghavi:
Of course.
Dr. Loretta Nastoupil:
If we just understand what that tumor is, and then we need to enrich our tissue banks and partner together where we understand more about those patient cases that don’t do as expected. So again, that can inform our next duration of trials.
Dr. Sanam Loghavi:
Absolutely. I think one thing that is really under-emphasized, is the importance of banking tissue. And I would say that even in a center like ours, where we have very, very good resources, we don’t routinely biobank everything, just because we don’t have the bandwidth and capacity. So I would say, maybe for administrators or people who fund hospitals, think about tissue banks, think about funding tissue banks, think about resources. Because really we’re going to learn so much if we have tissue available to work with. But if you don’t have tissue, it doesn’t matter what kind of technology you have, you’re not going to be able to do anything. So with that, we’ll end it. And it was a pleasure speaking with you today.
Dr. Loretta Nastoupil:
It’s a pleasure. Thank you.


















